Subtotal: $1.20
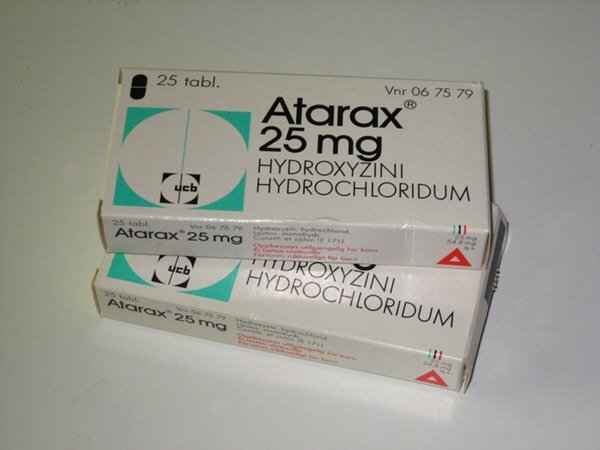
Atarax 25mg
$2.00 $1.60
Atarax 25mg (Hydroxyzine) is a drug which reduces the central nervous system’s activity and also acts as an antihistamine to reduce the body’s natural chemical histamine which produces skin hives and symptoms of sneezing and a runny nose.
PRICE/PILL
buy Atarax 25mg (Hydroxyzine) is indicated to assist in the management of anxiety in adults.
buy Atarax 25mg (Hydroxyzine) is indicated for the management of pruritus associated with acute and chronic urticaria, including cholinergic and physical types, and atopic and contact dermatitis in adults and children.
Posology
Atarax should be used at the lowest effective dose and for the shortest possible duration.
In adults and children over 40 kg in weight, the maximum daily dose is 100 mg per day.
Anxiety
Adults 50-100mg daily in divided doses
Pruritus
Adults Starting dose of 25mg at night increasing as necessary to 25mg three or four times daily.
Elderly
In the elderly, the maximum daily dose is 50 mg per day (see section 4.4). A reduced dose is advised. This is due to a possible increase in the volume of distribution, prolonged action and the possible effect of age-related changes on pharmacologic functions, including hepatic metabolism and renal excretion (see Section 5.2 ‘Pharmacokinetic properties’)
Paediatric Population
In children up to 40 kg in weight, the maximum daily dose is 2 mg/kg/day.
From 6 months to 6 years, 5-15mg daily in divided doses adjusted depending on the child’s weight.
In children and adolescents over 40 kg in weight the maximum daily dose is 100mg per day.
For children over 6 years, starting at 15-25mg and increasing to 50-100mg daily in divided doses adjusted according to the child’s weight.
As with all medications, the dosage should be adjusted according to the patient’s response to therapy.
Hepatic impairment
The total daily dose should be reduced by 33%. Use in patients with severe liver disease should be avoided (see Section 4.4 ‘Special Warnings and Precautions for Use’)
Renal impairment
For patients with moderate or severe renal impairment, it is recommended that the total daily dosage should be reduced by 50% (see Section 4.4 ‘Special Warnings and Precautions for Use’).
Method of administration: oral.
Atarax is contra-indicated in the following:
• patients who have shown previous hypersensitivity to hydroxyzine hydrochloride, cetirizine, other piperazine derivatives, aminophylline or ethylenediamine, or any of the excipients of Atarax (for a full list of excipients see section 6.1 ‘List of excipients’)
• Patients with a known acquired or congenital QT interval prolongation.
• Patients with a known risk factor to QT interval prolongation including a known cardiovascular disease, significant electrolytes imbalance (hypokalaemia, hypomagnesaemia), family history of sudden cardiac death, significant bradycardia, concomitant use with drugs known to prolong the QT interval and/or induce Torsade de Pointes (see sections 4.4 and 4.5).
• asthmatics who have previously experienced a serious anti-histamine-induced adverse bronchopulmonary effect
• porphyria
• pregnancy and breast-feeding (see section 4.6 ‘Fertility, pregnancy and lactation’)
Contains lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
Cardiovascular effects
Hydroxyzine has been associated with prolongation of the QT interval on the electrocardiogram. During post-marketing surveillance, there have been cases of QT interval prolongation and torsade de pointes in patients taking hydroxyzine. Most of these patients had other risk factors, electrolyte abnormalities and concomitant treatment that may have been contributory (see section 4.8).
Hydroxyzine should be used at the lowest effective dose and for the shortest possible duration.
Treatment with hydroxyzine should be stopped if signs or symptoms occur that may be associated with cardiac arrhythmia, and the patients should seek immediate medical attention.
Patients should be advised to promptly report any cardiac symptoms.
Patients with hepatic impairment
Due to its sedative properties, use of hydroxyzine should be avoided in severe liver disease due to an increased risk of coma, and in patients with hepatic failure due to possibility of hepatic encephalopathy.
Hydroxyzine elimination is impaired in patients with hepatic dysfunction secondary to primary biliary cirrhosis. Dosage should be modified for patients with hepatic impairment (see Section 4.2 ‘Posology and Method of Administration’)
Patients with renal impairment
Atarax should be used with caution in patients with impaired renal function (see Section 4.2 ‘Posology and Method of Administration’). It is uncertain whether the drug may accumulate or have other adverse effects in such patients. Atarax is completely metabolised and one of the metabolites is the active metabolite cetirizine. Cetirizine is renally excreted and clearance is reduced in patients with moderate renal impairment and on dialysis compared to normal volunteers.
Elderly patients
Hydroxyzine is not recommended in elderly patients because of a decrease of hydroxyzine elimination in this population as compared to adults and the greater risk of adverse reactions (e.g. anticholinergic effects) (see sections 4.2 and 4.8). In elderly patients, it is recommended to reduce the dose of hydroxyzine due to a possible increase in the volume of distribution, prolonged action, and the possible effect of age-related changes on pharmacologic functions, including hepatic metabolism and renal excretion (see Section 4.2 ‘Posology and Method of Administration’ and Section 5.2 ‘Pharmacokinetic properties’)
Because of its potential antimuscarinic actions, Atarax should be used with caution in patients suffering from angle-closure glaucoma, urinary retention, prostatic hyperplasia, or pyroduodenal obstruction.
Caution is required in patients suffering the following conditions:
• seizure disorders including epilepsy
• myasthenia gravis
• dementia
• decreased GI motility
• bladder outflow obstruction
• stenosing peptic ulcer
• patients with breathing problems (e.g. emphysema, chronic bronchitis)
• increased intraocular pressure
• hyperthyroidism
• cardiovascular disease
• hypertension
Dosage adjustments may be required if Atarax is used simultaneously with other CNS depressants or with drugs having antimuscarinic properties (see section 4.5 ‘Interaction with other medicinal products and other forms of interaction’).
The concomitant use of alcohol and hydroxyzine should be avoided (see section 4.5 ‘Interaction with other medicinal products and other forms of interaction’).
Treatment should be stopped for one week before skin testing for allergy is undertaken, and for 96 hours prior to a methocholine test.
Children and the elderly are more susceptible to side-effects.
Patients should be warned of impaired judgement and dexterity.


 Ephedrine tablet 30mg
Ephedrine tablet 30mg